July 19, 2022
Test
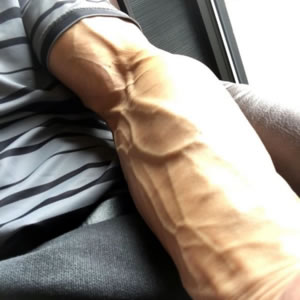
Fist Assist is a novel, non-invasive, external device which uses a pneumatic balloon for intermittent vein compression.
Vein dilation can assist in hemodialysis fistula development and phlebotomy procedures.
** For USA Fist Assist Arm Massager Customers: if you have any refund or return questions, please contact Airos Medical at www.fistassistusa.com
Changing the Standard of Vein Dilation
** The Fist Assist FA-1 device is cleared for sale in the USA as an arm massager and increase circulation device.
There is a need for simple, cost-reducing, patient empowering device to assist in global vein dilation.
How it Works
The Fist Assist device is an external medical intermittent pneumatic compression device that can apply intermittent pressure to a specific arm vein that may help dilate the vein for eventual AV fistula placement and blood draw.
The Problem:
- Over 400k patients in the US receive hemodialysis for end-stage renal disease (ESRD): 150k new patients start hemodialysis yearly.
- Hemodialysis costs an average of $89,000 per patient annually in the US; a total annual cost of $42 billion (significant portion is maturation and catheter issues)
- AV fistulas are the preferred method of vascular access for hemodialysis
- 70% of fistulas thrombose or require corrective procedures to maintain or restore blood flow within 1 year of initial surgery
- Basic science and clinical studies demonstrate the importance of vein enlargement
Chronic Kidney Disease: A Global Opportunity
1 in 10 Adults
Will have some form of chronic kidney disease (CKD)
40 Million Americans
Are currently classified as having CKD
3.6 Million Patients
Worldwide currently have stage 5 CKD or End Stage Renal Disease (ESRD)
4.9 Million Patients
Worldwide will be on dialysis by 2025
OUR MISSION:
Develop & produce a novel, non-invasive, low cost device to:
- Assist with Fistula Maturation
- Assist with Venous Pre-surgery preparation
- Decrease total catheter contact time and fistula development time
- Improve fistula maturation and cannulation
- Significantly reduce costs in treating Stage 4 and ESRD markets
- Empower patients to have a device for vein dilation and phlebotomy
Watch our video to learn more:
Innovative, Wearable, Intermittent Pneumatic Compression Device for Vein Dilation
01
wrap around arm
02
Press Start
03
See results!